por
Brendon Nafziger, DOTmed News Associate Editor | September 04, 2012
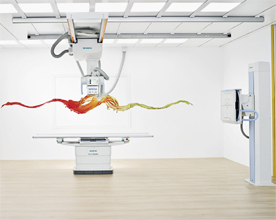
Multix Fusion (Credit: Siemens)
Siemens Healthcare said Tuesday that the digital version of its Multix Fusion X-ray system received Food and Drug Administration 510(k) clearance and is now for sale in the United States.
The new digital radiography system is being marketed as a "comparatively low" cost option, according to the company's promotional materials.
The DR Multix Fusion features a cassette-sized flat panel detector, a color touch display, a height-adjustable scanning table that can bear up to 660 pounds, and a nearly 6-foot-long telescopic column for the ceiling-mounted tube to be pulled down to reach patients' feet, Siemens said.
The model is a digital update on the film-based Multix Fusion that was
released in March by the Malvern, Pa.-based company.
