por
Brendon Nafziger, DOTmed News Associate Editor | August 02, 2011
Medtronic Inc. said Monday it received permission from the Food and Drug Administration to go ahead with a clinical trial to test the safety of its next generation MRI-compatible pacemaker.
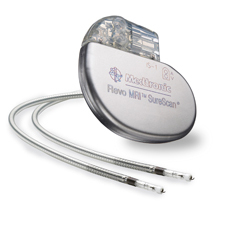
The Advisa DR MRI SureScan, available in Europe since last year, is the follow-up to the Revo MRI SureScan, which
got FDA approval in February, becoming the first pacemaker conditionally cleared for use around MRI scanners.
The prospective, randomized, trial will enroll 270 patients worldwide, and is expected to wrap up by May 2013, according to the study's Clinical Trials page. It will not be blinded.



Ad Statistics
Times Displayed: 75114
Times Visited: 5315 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
The goal of the trial will be to determine the safety and effectiveness of the device, and to see whether it affects the quality of the MRI image.
Dr. Edward J. Schloss, who implanted the first Advisa in a U.S. patient as part of the clinical trial at The Christ Hospital in Cincinnati, called the trial "an important milestone" for heart patients needing MRI scans.
Fridley-based Medtronic estimates 50-75 percent of patients with pacemakers will need an MRI at some point in their lives.