por
John R. Fischer, Senior Reporter | November 25, 2018
The FDA has cleared icobrain for
CT assessments of TBI
A new application could open up the potential for delivering personalized treatments to patients with traumatic brain injuries.
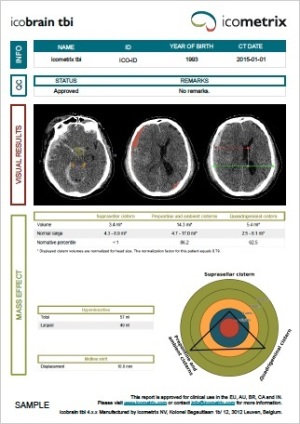
Following an additional clearance from the FDA, software developer Icometrix has equipped its icobrain product with icobrain tbi for CT images, a function that utilizes deep learning to assess CT images depicting TBI cases.
“The development of this product quantifying CT scans of TBI patients in routine clinical practice helps in the standardized interpretation of acute non-contrast CT scans.” Prof. Dr. Bart Depreitere, a neurosurgeon at UZ Leuven, said in a statement. “Up until today, TBI was always assessed with the naked eye, but this is now complemented by AI technology to provide valuable metrics following clinical guidelines for improved patient care.”



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
More than 50 million people experience a TBI incident annually, with experts estimating that approximately 50 percent of the world’s population will be subject to one or more TBI experiences during their lifetime. Conventional methods and technology are designed to carry out qualitative approaches, limiting the application of personalized treatment to individual cases.
The solution is the first CT product available for clinical assessments of TBI, and is capable of quantifying clinically important metrics, such as hyper-dense volumes, cisternal compression and midline shift to better characterize and manage TBI in acute clinical settings, similar to the main use of its MR image quantification software, which was
cleared by the FDA in 2016. It recently expanded upon its portfolio
to aid in the diagnosis of dementia.
“Our quantitative MR measurements in people with MS, dementia and TBI are used globally, and we are receiving a lot of positive feedback from clinicians, saying that the reports aid significantly in the treatment of their patients.” Dirk Smeets, VP of clinical for icometrix, said in a statement. “It is great to be able to provide a full clinical package with our CT and MR products for better patient care.”

