por
John R. Fischer, Senior Reporter | March 05, 2018
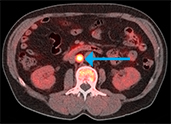
Axumin has been added to
the NCCN Clinical Practical
Guidelines in Oncology for
Prostate Cancer
The National Comprehensive Cancer Network (NCCN) has added Blue Earth Diagnostic’s Axumin Injection as one of the treatments recommended in its NCCN Clinical Practice Guidelines in Oncology for Prostate Cancer.
The addition of the PET imaging agent, designed for patients with suspected recurring prostate cancer, indicates it as a viable option in the clinical workup of patients with recurrence or progression of their prostate cancer, and provides more comfort among private payors in backing it as a form of treatment.
“CMS and Medicare already reimburse for the product across the country. Commercial payors are sometimes a little more reluctant to bring on new therapies, new imaging products certainly,” Peter Gardiner, chief medical officer for Blue Earth Diagnostics, told HCB News. “That’s been the case with us. They have begun to pay for the test when it is ordered by the physicians but there’s been reluctance in some quarters. Often the payors will use inclusion and NCCN guidelines as an additional stamp of the utility relevancy of a new technology, whether it’s drugs or imaging products like ours, which are regulated the same way a pharmaceutical therapy would be. That’s why NCCN is an important step forward, because it will facilitate, we believe, that process of reimbursement and payment by the private payors.”



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
Many physicians and health care providers rely on NCCN guidelines as a benchmark for assessing clinical utility of different treatments options.
The addition of Axumin builds upon its level acceptance, including its
May 2016 clearance by the FDA as the first F-18-labeled PET imaging agent for patients with suspected recurrent prostate cancer.
It also
receivedTransitional Pass-Through Status in the Hospital Outpatient Prospective Payment System 2017 Final Rule from the Centers for Medicare & Medicaid Services (CMS) in January 2017, enabling its use among Medicare and Medicaid users.
Blue Earth Diagnostics CEO Jonathan Allis says there are currently 18 facilities in the country that produce Axumin, enabling a reach of about 60 percent of the U.S. population, and that the addition of NCCN guidelines goes hand-in-hand with its plans for growing these numbers.
“This year we will increase the number of manufacturing facilities to at least above 30 and we think that gets us into about 80-90 percent of the U.S. population," he told HCB News. "I think having Axumin appear in the NCCN guidelines makes us feel more confident to continue to invest in rolling out the supply chain, because we think that there will be more coverage of the product by payors and therefore, it’s a good business case to continue to increase supply across the country as well.”
Blue Earth Diagnostics recently
partnered with GE to manufacture Axumin as a PET imaging agent in the U.K. and with Seibersdorf Laboratories
to produce supplies of it for Austria, the Czech Republic, Croatia, Germany, Hungary, Slovakia and Slovenia.

