Lunit AI to be deployed in Philips X-ray systems
por
John R. Fischer, Senior Reporter | March 05, 2021
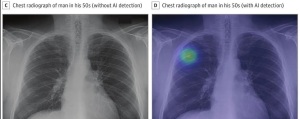
Lunit Insight CXR will now be available in Philips' diagnostic X-ray systems
Lunit Insight CXR, an AI software designed by startup Lunit and used to assess chest X-rays, can now be found as a component in Philips’ diagnostic X-ray solutions.
The two companies announced the integration and their newfound partnership at the European Congress of Radiology virtual event.
“In times of higher patient throughput and increasing pressure to reduce costs, AI-based early detection and reading support can help drive faster decision-making about follow-up procedures and time to diagnosis. We also see a significant demand for early notification and decision-making at the point of acquisition directly at the modality, helping to improve the entire diagnostic imaging chain,” Daan van Manen, general manager of DXR (Diagnostic X-Ray) at Philips, told HCB News.
Lunit Insight CXR chest detection suite is designed to identify 10 of the most common findings in a chest X-ray by mapping their location. It then shows scored calculations of the actual existence of a finding for accurate analysis. These findings include atelectasis, calcification, cardiomegaly, consolidation, fibrosis, mediastinal widening, nodule, pleural effusion, pneumoperitoneum, and pneumothorax.
It also can prioritize abnormal cases for faster triage and has an accuracy rate of 97% – 99% that has been validated in major publications. The solution will be made available for new products released by Philips and within its installed base of MobileDiagnost wDR, DigitalDiagnost, DigitalDiagnost, ProxiDiagnost N90 as well as CombiDiagnost R90 systems. Installations will not require costly modifications/upgrades for these existing systems.
For Philips, the partnership extends its AI sector further into precision diagnosis and enables it to provide better outcomes, improve patient and staff experience, and lowers cost of care. The deployment of Lunit Insight CXR is expected to help providers avoid complex IT projects and help lower barriers for adopting AI.
“Technologies providing more user-independent outcomes on a consistently high quality level can help caregivers stay on the clinical edge while managing costs,” said van Manen.
Lunit INSIGHT CXR is CE marked and clinically available in Europe, the Middle East, Latin America, South East Asia, Australia, and New Zealand. It is expected to be cleared by the FDA in 2021.
|
|
|
You Must Be Logged In To Post A Comment
|
