Philips releases ultimate ultrasound solution for breast assessment
por
John R. Fischer, Senior Reporter | November 01, 2018
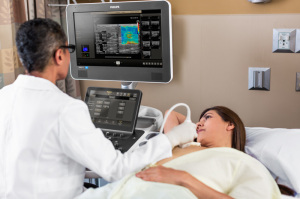
Philips releases its ultimate ultrasound
solution for breast assessment
A new addition to the Royal Philips ultimate ultrasound family has entered the U.S. marketplace.
The Dutch-based healthcare giant launched its ultimate ultrasound solution for breast assessment, combining high-quality imaging with complementary tools tailored for breast screenings to assess, monitor and treat such diseases with greater diagnostic confidence, especially in patients with dense breast tissue. Available with the Philips EPIQ and Affiniti ultrasound systems, the solution utilizes anatomical intelligence to enhance reproducibility and streamline workflow.
"Anatomical intelligence for breast allows clinicians to visually map and annotate screened anatomy with minimal user interaction while documenting full coverage of the breast, eliminating the need to perform repeated scans in specific areas," Jeff Cohen, VP of general imaging and Women’s healthcare ultrasound business segment, Philips, told HCB News.
The feature provides a comprehensive view of the scanned area not only during the acquisition process, but after its completion, for further review and assessment.
Any challenges that arise during this process are addressed by an eL18-4 ultra-broadband linear array transducer, which utilizes PureWave crystal technology. The component enables the transducer to deliver detailed resolution and uniformity of the breast tissue, and provides an extended depth of field performance that does not sacrifice image quality and allows the the system to image even those who may be more technically challenging. In addition, both the eL18-4 and PureWave are able to scan any patient body type for detailed assessments, regardless of tissue density or mass, requiring no need to switch between tools.
The system is able to assess a wide array of breast lesions with greater diagnostic confidence through a combination of ElastQ Imaging shear wave and strain elastography, establishing full-solution elastography to collect more definitive information on breast tissue stiffness.
It also is equipped with new precision biopsy technology which reduces blind zones and enhances needle reflections for more targeted biopsies that improve the patient experience.
The integration of all four features reduces total exam time and enhances the patient experience while still being able to perform necessary ultrasound exams to confirm the presence of lesions in dense breast tissue. The condition often prevents mammograms from detecting tumors early on and is carried by an estimated 43.3 percent of women between 40 and 74, according to a report in the Journal of the U.S. National Cancer Institute. With the ultimate ultrasound solution for breast assessment, patients can avoid multiple appointments, and inconvenient room or equipment changes.
"This new solution for breast assessment was developed to provide clinicians with the tools they need to efficiently assess, monitor and treat breast diseases, all in one place," said Cohen. "It delivers high-quality imaging, tailored breast exam tools, and enhanced workflow features to help clinicians deliver the best care for their patients."
Its release follows the launches of other ultimate ultrasound solutions, including one for liver assessment and another for small parts imaging for cases involving breast, testicle, thyroid and musculoskeletal injuries.
The system is FDA cleared and CE marked.
|
|
|
You Must Be Logged In To Post A Comment
|
