Hospira scores CE mark for Nuitiv, needle-free IV connector
por
Lauren Dubinsky, Senior Reporter | January 11, 2016
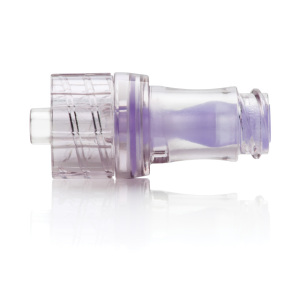
Hospira's Nuitiv
needle-free IV connector
Hospira has received CE mark to commercially distribute its Nuitiv clear, needle-free intravenous (IV) connector in the UK and France. The system is expected to help reduce the risk of catheter-related bloodstream infections, which represent a costly and highly common form of health care-acquired infection.
“Needle-free connectors like Nuitiv are designed to enable the safe delivery of IV medication and support the reduction of catheter-related bloodstream infections, also known as CRBSIs, which are the most common cause of health care-associated infection to the bloodstream,” Julie Sawyer Montgomery, vice president of device global marketing and international sales at Hospira, told HCB News.
Every year, central venous catheters can cause about 80,000 CRBSIs in the ICU, and one incident of CRBSI can cost about much as $56,000 to treat, which includes the cost of pharmacy charges, catheter changes, lab tests and an additional day in the ICU, according to the WHO. It is also the most common type of health care-associated infection of the bloodstream.
Nuitiv is able to be needle-free because the male luer is connected from a device such as a syringe or IV set, which activates the valve and enables fluid to flow. Its connector’s clear housing gives clinicians a better view of the patient’s fluid pathway and helps ensure it’s free of particulate, and it has a smooth septum surface for disinfection.
“A smooth external septum surface with few if any gaps can be more thoroughly disinfected, which facilitates cleaning and compliance with contamination prevention and aseptic technique best practices,” wrote Sawyer Montgomery.
Nuitiv is also currently available in Chile and will be launched commercially in Europe, Latin America, the Middle East and across the Asia-Pacific region in the near future.
Hospira is not the only manufacturer with a needle-free IV connector in its portfolio. ICU Medical, Inc., Baxter, B. Braun Medical, Inc., and CareFusion are a few of the other manufacturers that offer similar solutions.
Needle-free IV connectors are used in almost all IV administrations of patient medication and they represent a critical component of the $1.9 billion global IV consumables market, according to Hospira.
|
|
|
You Must Be Logged In To Post A Comment
|
