por
John R. Fischer, Senior Reporter | March 18, 2024
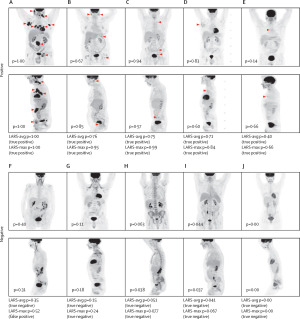
LARS, a deep learning model, was 90% accurate at identifying signs of lymphoma.
In one of the largest studies to date and one that took several years to complete, an AI-assisted image analysis computer model designed by Sweden- and New York-based researchers accurately detected symptoms of lymph node cancer (lymphoma) from PET scans in 90% of cases.
Ida Häggström, associate professor in the department of electrical engineering at Chalmers University of Technology, says that lymphoma is a rare cancer, meaning that radiologists are less likely to have interpreted such cases.
Working with clinical researchers at Memorial Sloan Kettering Cancer Center, she developed Lars (Lymphoma Artificial Reader System), a deep learning system trained to find patterns in PET scans that align with positive or negative findings for lymphoma to make the best possible prediction and help reduce errors resulting from lack of familiarity in interpreting the condition in scans.
Trained on more than 17,000 images from over 5,000 lymphoma patients, the AI model is not programmed with predetermined instructions and instead teaches itself which image patterns show signs of the disease. The study on its efficacy is called
Deep learning for [¹⁸F]fluorodeoxyglucose-PET-CT classification in patients with lymphoma: a dual-centre retrospective analysis.
"I have used what is known as supervised training, where images are shown to the computer model, which then assesses whether the patient has lymphoma or not. The model also gets to see the true diagnosis, so if the assessment is wrong, the computer model is adjusted so that it gradually gets better and better at determining the diagnosis,” said Häggström in a statement.
Model of how LARS extracts and analyzes PET imaging data.
To ensure its accuracy, she and her colleagues adapted the model to distinguish between cancer and the temporary treatment-specific changes that can be seen in images following radiotherapy and chemotherapy. During the study, they used and improved it through an analysis of image archives stretching back more than ten years, comparing the patients’ final diagnoses with PET and CT scans before and after treatment and using the information to train the model. They said its accuracy was especially relevant in difficult-to-interpret cases.
More work is required to validate the model before it can be applied in clinical practice. Häggström says the model has been made available for use by other researchers to build on the findings around its efficacy, as the clinical tests required are extensive.
The findings of the study were published in
The Lancet Digital Health.
