por
John R. Fischer, Senior Reporter | May 17, 2021
AIR Recon DL is now available on GE's SIGNA 7.0T MR scanner
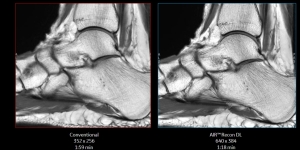
GE Healthcare’s deep learning image reconstruction technology, AIR Recon DL, is now available on its SIGNA 7.0T MR scanner.
The FDA greenlit the integration of the two technologies, with AIR Recon DL poised to improve image quality for diagnostic confidence and decrease scanning time.
“AIR Recon DL — which has received overwhelmingly positive user feedback and fast, broad adoption — further extends the capabilities of GE Healthcare’s completely new SIGNA 7.0T system, empowering our research and clinical partners to pursue new frontiers in neuroscience and musculoskeletal imaging,” said Jie Xue, president and CEO of MR at GE Healthcare, in a statement.



Ad Statistics
Times Displayed: 75267
Times Visited: 5317 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
AIR Recon DL utilizes all raw data collected by MR scanners to maximize image quality and resolution, even when scanning time is shorter. The solution first
launched on GE Healthcare’s 3.0T and 1.5T MR scanners and has been found to create sharper and less noisy images as well as reduce exam times by 30% to 50%.
Used for a broad range of neurological and musculoskeletal disease applications, SIGNA 7.0T can produce high-resolution imagery with attention to detail for research and clinical work on image anatomy, function, metabolism and microvasculature in the brain and joints. The 60-centimeter bore scanner is equipped with UltraG gradient technology; GE’s whole-body gradient coil with 113mT/m and 260 T/m/s for ultrahigh-field imaging speed demands; contrast, resolution; advanced diffusion; and functional brain imaging. It was
approved by the FDA in November 2020 and is expected to help with imaging research for disorders such as Alzheimer’s disease and mild traumatic brain injury.
“AIR Recon DL for SIGNA 7.0T takes everything we love about the ultrahigh-field 7.0T’s strength, namely, its ability to visualize high-resolution tissue structures, and brings it to the next level by reducing noise and edge ringing,” said Dr. Garry Gold, professor of radiology at Stanford and clinical reviewer of GE Healthcare’s FDA application. “As a result, AIR Recon DL with 7.0T helps reveal a more complete picture — providing greater clinical insight for improved patient outcomes and opening up new opportunities for research across various care areas.”
AIR Recon DL is expected to enable more clinical translation of research and development between GE Healthcare MR scanners, from the ultrahigh-field 7.0T to more commonly available 1.5T and 3.0T scanners in clinical settings.
AIR Recon DL is developed on GE Healthcare's Edison Intelligence platform and is available as an upgrade or with new system purchases.

