por
John R. Fischer, Senior Reporter | February 11, 2020
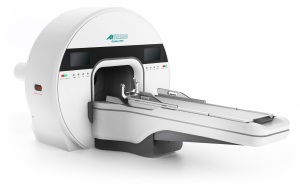
The FDA has said yes to the sale of Akesis’ advanced gamma stereotactic radiosurgery system (SRS), the Akesis Galaxy RTx, in the U.S.
The solution is the second system in the Akesis Galaxy SRS platform to score FDA clearance, and will equip clinicians with a combination of gamma-based therapy and continuous 360º rotational technology to optimize treatment planning and delivery for intracranial radiosurgery procedures.
“We are very pleased that the Akesis Galaxy RTx system will now be available in the United States,” said Akesis chief executive officer Ian Dickson in a statement. “By combining continuous rotational technology with the largest choice of automated collimator selections, clinicians have virtually infinite possibilities to shape the dose distribution. They now have the flexibility to build more complex composite shots and can ‘shrink-wrap’ the dose around the target.”




Ad Statistics
Times Displayed: 30380
Times Visited: 759 Stay up to date with the latest training to fix, troubleshoot, and maintain your critical care devices. GE HealthCare offers multiple training formats to empower teams and expand knowledge, saving you time and money
The rotational delivery of the system offers flexibility for shaping dose distribution, compared to traditional, fixed sector-based delivery. Its <0.5mm accuracy enables strong dose fall-off and makes it potentially better for sparing organs at risk.
It also has a source arrangement of just 30 gamma sources in a compact drawer that reduce the cost of ownership as well as downtime during source replacement. Its design is based partially on well-proven isometric design principles published in more than 2,000 peer-reviewed papers for Co-60-based radiosurgery.
Akesis previously
granted a sneak peak of another Galaxy system, the Akesis Galaxy RTi, which is also a gamma stereotactic radiosurgery system equipped with continuous 360 degree rotational technology. The solution is built to provide real-time, in-line CBCT and kV/kV imaging for improved efficiency and confidence in treatment delivery, and is made with only 30 gamma sources too. It was showcased at the American Society for Radiation Oncology 2019 Annual Meeting.
Akesis Galaxy RTx is geared toward high-throughput institutions and smaller cancer centers, and is expected to integrate well within value-based reimbursement models, according to Akesis.