por
John R. Fischer, Senior Reporter | April 27, 2018
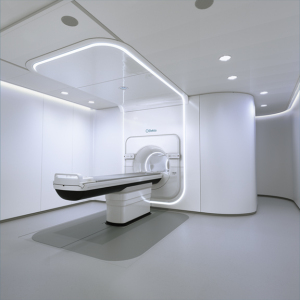
Proton Partners International (PPI) Ltd. has agreed to purchase and install Elekta’s MR-linac systems at a network of cancer centers under development in the U.K.
The British healthcare enterprise plans to acquire five systems for an order value of £25 million ($34,915,950) and begin installation of the first in about 12 months. Installation of all five is expected to take place over a period of three years.
“Elekta’s MR-linac gives physicians the power to see while they treat at the time of delivery, enabling truly personalized radiation therapy regimens,” Kevin Brown, global vice president of scientific research at Elekta, told HCB News. “With Elekta’s MR-linac, tumors and surrounding tissues can be located and their movement tracked with unsurpassed accuracy at the time of treatment. This allows treatment plans to be adapted while the patient is on the table in response to changes in tumor position, shape, biology and the relationship to sensitive organs over time.”



Ad Statistics
Times Displayed: 119604
Times Visited: 6905 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
Elekta’s MR-linac system is the only radiotherapy solution that integrates an advanced linear accelerator and high-field, 1.5 Tesla MR imaging without compromising either system.
It is the first to deliver radiation and provide fast acquisition of high-quality, high-field MR images simultaneously, creating a “see what you treat” practice during treatment that allows for clinicians to respond based on what they see in real time.
In addition, Elekta has
integrated additional imaging capabilities, such as diffusion-weighted imaging for dose painting, and to take more time to validate its linac control system for optimal preparation in the potential release of dynamic, online adaptive treatments, pushing back its expected time frame for CE approval to do so.
“This radical improvement – “see while you treat” – will allow clinicians to be more confident that they are accurately and precisely dosing the target area at all times, helping to achieve the goal of potential reductions in treatment margins around the tumor, to spare more healthy tissue,” said Brown. “It may also allow clinicians to deliver a higher dose of radiation per fraction to the tumor, which may improve treatment outcomes or reduce the number of treatment sessions.”
Previous installations of the system on British shores have
taken place at the U.K. Institute of Cancer Research and The Royal Marsden;
and The Christie NHS Foundation Trust.
It has also been
set up at The Froedtert & Medical College of Wisconsin (MCW) Clinical Cancer Center;
at The Netherlands Cancer Institute (NKI); and
at the Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto, with plans to
install another at Memorial Sloan Kettering Center in New York at the end of 2018.
The solution is still a work in progress and is not available for sale.
The agreement is pending approval from each company’s board of directors.