por
John R. Fischer, Senior Reporter | April 25, 2018
ViewRay showcases developing
enhancements for MRIdian Linac
radiotherapy system at ESTRO
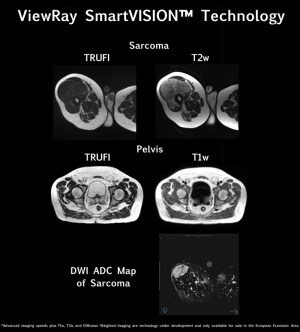
MR pulse sequencing is one in a series of enhancements that ViewRay Inc. showcased this weekend in its MRIdian’s SmartVision MR image guidance technology at ESTRO 2018 in Barcelona, Spain.
The Ohio-based enterprise plans to develop the upgrades to heighten the bone and soft tumor visualization capabilities of its MRIdian Linac radiotherapy system to ensure greater precision in radiation delivery. News of the enhancements follows the recent implementation of the solution earlier this month at the University Clinic Heidelberg in Germany.
"We are grateful to the German Research Foundation (DFG) for granting us the opportunity to utilize such a cutting-edge instrument to investigate the benefits of soft-tissue visualization and real-time plan adaptation to improve our patient treatment,” medical director and professor Jürgen Debus, head of the MRIdian Linac program and the Heidelberg Heavy Ion Center, said in a statement upon the completion of the installation.
The solution will utilize T1w and T2w MR pulse sequences for the development of visuals, as well as enhanced contrast between malignant and healthy tissue.
Further assistance will potentially be available with the integration of diffusion-weighted imaging for distinguishing between tumors and normal tissue; and for the possible assessment and prediction of tumor response to radiotherapy.
The integration of these enhancements is set to double the speed of MRIdian’s SmartVISION’s MR imaging from four to eight frames per second. The technology will also provide two times higher image resolution and double improvement in MR signal-to-noise ratio (SNR), producing brighter and more detailed anatomical imaging.
In addition, the University Clinic Heidelberg will
research the system's potential for enhancing dose delivery through MR-guidance. Another partner, the University Hospital Zurich, will
investigate response prediction and assessment.
The enhancements are under development and only available for sale in the European Economic Area.
The first generation of the solution
is FDA cleared and can be purchased by U.S. consumers.
ViewRay Inc. did not respond for comment.
