por
John R. Fischer, Senior Reporter | March 01, 2018
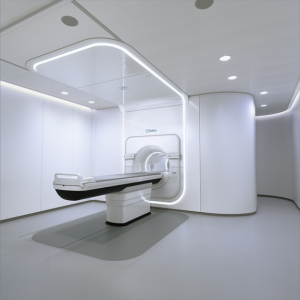
Memorial Sloan Kettering Cancer Center (MSK) has agreed to become a site of research for Elekta’s MR-linac system.
The New York City-based provider will utilize MR-linac’s capabilities to investigate new treatment standards, imaging protocols, and treatment planning methodologies for a variety of cancer indications.
“Memorial Sloan Kettering Cancer Center (MSK) is an ideal partner for Elekta to collaborate with as we continue developing the clinical protocols for our MR-linac technology,” Kevin Brown, global VP Scientific Research at Elekta, told HCB News. "Elekta is honored that MSK should choose our MR-linac to further their world-class research program.”



Ad Statistics
Times Displayed: 119604
Times Visited: 6905 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
The solution was
installed this past summer at at the Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto, and at
The Christie NHS Foundation Trust in the U.K. in October, 2016.
The establishment of the Elekta-MSK collaboration further builds upon evaluations conducted by the Elekta-MR linac Consortium, an international group of cancer centers whose founding members assisted in developing the solution.
Testing will focus on the use of the Elekta MR-linac for treating tumors in nine parts of the body that are difficult to address with conventional radiotherapy approaches, with the hope that the adaptive radiation capabilities of the MR-linac will go a long way in treating these masses.
“The ability to visualize the tumor during treatment and react to changes in the position and shape of the tumor in real time should enable higher radiation doses to be delivered to the tumor, while lowering the exposure of healthy adjacent tissues and organs at risk," explained Brown.
MSK's research MR-linac will be booked in the fourth quarter of Elekta's 2017-2018 fiscal year.
Data from these studies will be presented at the European Society for Radiotherapy & Oncology from April 20-24.
Elekta expects to receive
CE marking for the MR-linac in midsummer, the time frame of which was pushed back from the end of 2017 for the addition of more functional imaging capabilities and the opportunity to further validate its linac control system.