por
Lauren Dubinsky, Senior Reporter | February 28, 2018
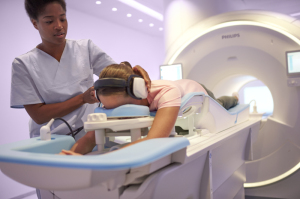
Philips' Ingenia Elition 3T MR
Royal Philips unveiled the Ingenia Elition 3T MR system at the ECR 2018 annual meeting in Vienna, Austria today.
“It’s attempting to respond to the dynamics of the health care system on a global basis,” Rob Cascella, executive vice president of the diagnosis and treatment businesses at Philips, told HCB News. “Today our problems are that imaging has gotten much more sophisticated, the staff is overworked and there are not more radiologists yet we have more information.”
The Ingenia Elition features Philips’ Compressed SENSE acceleration technology, which can reduce average scan times by about 50 percent, while maintaining image quality.



Ad Statistics
Times Displayed: 75267
Times Visited: 5317 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
“What we are aiming for is to help them do more patients in the same amount of time by simplifying the setup and scanning requirements of the machine,” said Cascella.
The system is also equipped with new patient-sensing technology and AI-driven SmartExam analytics that automatically plan, scan and process the exams. Many AI tools are for clinical decision support, but these new tools address operation and workflow.
The VitalScreen is a new user interface for workflow optimization that fully guides patient setup. VitalEye technology and algorithms process more than 200 body locations to spot signs of breathing — as a result, routine exam set-up time takes less than a minute.
Ingenia Elition also comes with a department operation effectiveness tool called Performance Bridge that assists with machine loading, as well as better magnet selection and patient scheduling.
“It covers everything from before the patient enters the department to when they leave the department, in terms of understanding that workflow around that patient and how to optimize it,” said Cascella.
The system is currently pending FDA approval and CE mark. To date, it has been installed in the Amsterdam Medical Center and the Technical University of Munich — both of which have helped with the development and validation process.

