por
John R. Fischer, Senior Reporter | October 19, 2017
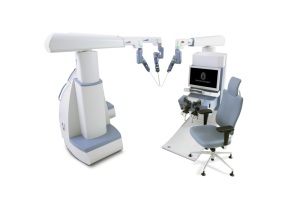
Senhance Surgical Robotic System
TransEnterix, Inc.'s Senhance Surgical Robotic System will soon be available to U.S. consumers for abdominal surgery, following its recent approval by the FDA.
The system’s clearance marks the first entrant into the market of abdominal robotic surgery since 2000, and provides physicians with greater comfort and control while reducing the invasiveness of procedures.
“The surgical robotics field has advanced over the last two decades by offering solutions that provide minimally-invasive access to difficult-to-reach anatomy, precise instrument control, and the ability to visualize in these tight spaces,” Todd Pope, president and CEO of TransEnterix, told HCB News. “However, broader application of robotics has been limited by higher per-procedure costs and the ability to convert open surgery to robotic surgery. The Senhance brings a new solution to the current limitations of robotic surgery, opening up robotic applications to high volume, multispecialty laparoscopic surgeries.”
Laparoscopic procedures using basic tools limit the capabilities, control and comfort of surgeons during procedures. Senhance heightens these qualities by allowing physicians to direct small surgical instruments through the use of a camera with precise movements.
The system consists of technology that enables haptic feedback and eye-sensing camera control and is the first robotic surgery platform to offer these features in the fields of digital laparoscopy and robotics. It also provides an open platform strategy, enabling physicians and providers to leverage evolving technology and existing investments in the OR, and uses reusable instruments, making robotic surgery cost-effective on a per-procedure basis.
TransEnterix revealed the
brand name for the system in September 2016 and has since launched commercial operations in
Germany and begun clinical use of it in
France.
Pope says Senhance will provide enhancements that can be scaled across high-volume procedures, specialties and different types of hospitals.
“Our vision is that there should be a Senhance System in every OR that is focused on high-volume minimally-invasive surgery, similar to how all hospitals have core technology, like MRs and CT scans,” he said. “Why should only some hospitals and some patients benefit from robotic technology? This is now something transferable on a broad scale that meets today’s economic constraints. Digital laparoscopy should become the new standard.”
The system is also FDA approved for laparoscopic colorectal surgery as well as gynecologic surgery. It is CE-marked.
