por
Lauren Dubinsky, Senior Reporter | September 22, 2017
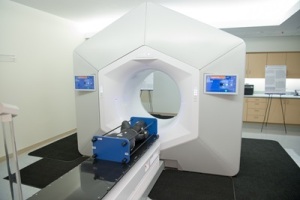
Penn Medicine recently became the first to treat a patient with the new Varian Halcyon radiation treatment platform.
"The technology brings the opportunity for increased efficiency, reduced time on the treatment machine for patients, and improved treatment plans in many cases," Dr. James Metz, chair of radiation oncology at Penn, told HCB News.
Penn partnered with Varian in 2015 to develop and validate the platform. A team of physicians, physicists therapists and dosimetrists spent about two years evaluating and testing it.




Ad Statistics
Times Displayed: 39212
Times Visited: 1059 Stay up to date with the latest training to fix, troubleshoot, and maintain your critical care devices. GE HealthCare offers multiple training formats to empower teams and expand knowledge, saving you time and money
"We have evaluated the technical delivery and clinical implementation opportunities in multiple disease sites and developed techniques for utilizing this new technology efficiently in the clinic," said Metz.
The platform involves only nine steps instead of the 30-plus that are required for conventional radiation therapy platforms. Testing showed that the Varian Halcyon delivers radiation as effectively as traditional platforms.
It's also twice as fast as other platforms, which means that patients spend less time undergoing each treatment session. For the first patient treated, it only took 13 minutes to set up the therapy room, capture images, deliver the therapy and break the equipment down.
The patient was under the beam for three minutes. In a typical scenario, the whole process would have taken more than 20 minutes including 10 minutes of beam time.
"We believe the patient experience overall will be improved dramatically due to the reduced time on the machine, many times with uncomfortable immobilization in place," said Metz.
He added that the platform can significantly reduce operational costs since the treatment is so efficient. The unit has built-in self-shielding so it requires one-third the wall thickness for radiation protection and half the power requirements compared to conventional linear accelerators.
In addition, the installation and commissioning time has been reduced from about two months to seven to 10 days.
Penn Medicine has already evaluated a number of disease sites including breast, cervix, head and neck and palliative treatments, and developed clinical techniques for safe delivery.
In the coming months we will extend this to multiple other disease sites to expand the things treated with this new machine," said Metz. "We expect to be able to treat almost everything we currently do with a conventional linear accelerator, with this machine."
Back to HCB News