por
Brendon Nafziger, DOTmed News Associate Editor | September 12, 2011
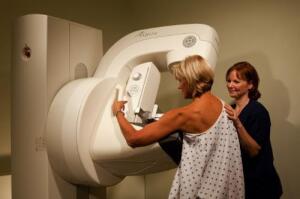
Aspire HD
(Credit: PRNewsFoto/FUJIFILM
Medical Systems U.S.A., Inc.,
Richard Freeda)
Fujifilm Medical Systems USA Inc. said Monday its full-field digital mammography system, Aspire HD, received the Food and Drug Administration's 510(k) clearance this month.
Known as Amulet outside the United States, the system already has 200 installs in Europe, according to the company.
The DR-based device offers 50-micron resolution and the company's new Direct Optical Switching technology, allowing direct image transfer. This results in more efficient image capture, producing studies with less noise and possibly lower dose, the company said.




Ad Statistics
Times Displayed: 30157
Times Visited: 744 Stay up to date with the latest training to fix, troubleshoot, and maintain your critical care devices. GE HealthCare offers multiple training formats to empower teams and expand knowledge, saving you time and money
Fujifilm now says it's the only vendor to sell both CR- and DR-based digital mammography units in the U.S. It introduced the first CR-based full-field digital mammography product to U.S. markets in 2006.
The Stamford, Conn.-based company says it has 8,000 full-field digital mammography systems installed worldwide.
The FDA only eased the approval process for full-field digital mammography systems
last November.

