por
Brendon Nafziger, DOTmed News Associate Editor | September 03, 2009
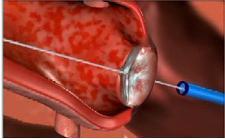
Computer simulation of the surgery
using Edwards Lifesciences'
transcatheter valve
Doctors in New Orleans replaced an 82-year-old man's heart valve without open heart surgery, according to a statement from Ochsner Health System released last week.
The team, led by cardiologist Stephen Ramee, M.D., inserted a new kind of transcatheter valve, called the Edwards SAPIEN aortic transcatheter heart valve, into an artery in the man's leg, and then threaded it up to his heart, according to Ochsner.
The operation was one of the last of the 2600 PARTNER (Placement of AoRTic traNscathetER valves) trials being conducted for the FDA on behalf of Edwards Lifesciences, the makers of the device.




Ad Statistics
Times Displayed: 48718
Times Visited: 1521 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
"It's like an angioplastic technique," Dr. Ramee tells DOTmed News. "You basically put a wire in the [blood] vessel, the wire acts as a guide, and guides the wire and the balloon up the leg to the heart."
The SAPIEN heart valve is designed for patients whose aortic valves have calcified or narrowed, but who are too sick for surgery.
"Calcific aortic stenosis is fatal without a valve replacement," Dr. Ramiee says. "Once the patient starts having those symptoms," he says, including chest pains, passing out and shortness of breath, "the patient is dead at two years" without treatment.
But the problem, according to Dr. Ramee, is that most patients with aortic stenosis are too weak to put under the knife. Patients with a blocked aortic valve are usually around 65 and many are in their 80s and 90s, he says, and they often have other complications. "They're not good patients," he says, "to put under anesthesia, and open their chests. If you recognize the condition, and they're not a candidate for surgery, they don't have good outcomes."
But with the Edwards SAPIEN heart valve, Dr. Ramee thinks patients too sick for open heart surgery might have a chance. "We're hoping they can die from something else," he says.
He does note, however, that because of the large size of the stent used to deliver the valve, it cannot be used on people with small veins, or whose leg arteries have blockages or calcium buildup. But he predicts that once it goes into general use, it will become much smaller. "A third smaller within a year," he says.
The experimental procedure was performed at 26 centers nationwide, some of which are still offering the treatment pending the FDA's verdict. Final results from the study are expected in six to 12 months, according to Dr. Ramee. In a statement published on Edwards Lifesciences' website, they said they would present new clinical evidence for the valve at the Transcatheter Cardiovascular Therapuetics conference in San Francisco later this month.
Read related news about Edwards, along with DOTmed's archive of reporting at "DM 10102". Just enter the story number into any search box on DOTmed.

