by
John R. Fischer, Senior Reporter | June 09, 2023
Ezra Flash (Photo courtesy of Ezra)
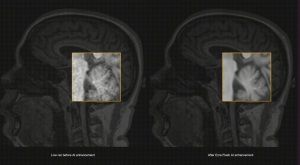
Healthcare startup Ezra is launching what it says will be the world’s first 30-minute full-body MR scan, following the FDA’s clearance of its proprietary AI technology, Ezra Flash for brain imaging.
The company, which formed in 2019, aims to detect over 500 conditions, (including cancer) in up to 13 organs for early-stage diagnoses. It says its scans, which use no IV contrast agents, have identified potential cancer in 13% of its members and noncancerous conditions in almost three-quarters.
Since Ezra Flash reduces the typical one hour scan to half an hour, the company is reducing the cost by 30%, from $1,950 to $1,350.



Ad Statistics
Times Displayed: 46606
Times Visited: 1410 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
While the faster and more affordable scans may come as good news to patients seeking preemptive insight on their anatomy, no medical society currently recommends full body MR scans, with the consensus among them being that there is a lack of evidence to justify these screenings for patients with no clinical symptoms.
"The ACR is concerned that such procedures will lead to the identification of numerous non-specific findings that will not ultimately improve patients' health but will result in unnecessary follow-up testing and procedures, as well as significant expense," according to an April 2023 statement on its website. "The ACR will continue to monitor scientific studies concerning the utility of screening total body MR."
Emi Gal, founder and CEO of Ezra, says longitudinal monitoring is necessary for detecting cancer early and minimizing unnecessary follow-up testing. He recommends radiologists have a patient’s prior scans on hand to determine if follow-up procedures are appropriate, as well as to minimize false positives in the long term.
"Radiologists looking at Ezra scans compare any concerning findings to what may be found on a member’s previous scans," he said. "The lack of change from one scan to another rules out many concerning conditions."
Gal also points out that guidelines around screening are always behind state-of-the-art innovations in clinical practice. "Screening mammograms were already routinely used in clinical practice as early as the 1970s, but it wasn’t until 1992 that Congress enacted the Mammography Quality Standards Act (MQSA) recommending mammograms for women over 45. That’s a 20 year gap between clinical practice and mandated guidelines."
Ezra Flash is trained on hundreds of thousands of proprietary longitudinal MR images from patients and healthy individuals. During its development, the algorithm was validated by five radiologists on its qualitative and quantitative performance, with the group helping to choose an AI model that could enhance MR images without obscuring any existing pathology.
"Our approach is to recommend a follow-up diagnostic scan of the organ of interest prior to any invasive procedure such as a biopsy," said Gal.
Ezra has reportedly scanned over 5,000 people and hopes to publish its first study in the next year demonstrating the sensitivity and specificity of full-body MR, and why it is an effective tool for screening asymptomatic patients.
It also plans to continue investing in AI to reduce scanning time to as little as 15 minutes in the next two-to-three years, and charge $500 for these exams. Additionally, it will apply AI to speed up interpretation times and eliminate operational inefficiencies.
The company has locations in New York, Los Angeles, San Francisco, Miami, and Las Vegas, and plans to roll out in more cities in the second half of 2023.

