Michigan hospital first in U.S. to use the Tryton Side Branch Stent
June 07, 2017
by Lauren Dubinsky, Senior Reporter
St. John Hospital and Medical Center in Michigan became the first in the U.S. to treat a high-risk coronary artery disease patient using the newly approval Tryton Side Branch Stent.
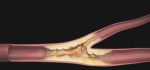
CAD patients often experience bifurcation, which is a buildup of plaque at the site where one artery branches from another. The standard treatment involves placing a stent in the main branch, but the side branch is usually not stented, leaving it susceptible to complications like occlusion.
"Bifurcation blockages are somewhat more challenging for cardiac interventionalists to treat than blockages that do not involve side-branch vessels, because current stents do not come in a "Y" configuration,” Dr. Antonious Attallah, cardiologist at SJH&MC who performed the procedure, said in a statement.
The Tryton Side Branch Stent, which received FDA approval in February, was designed to provide complete lesion coverage. Tryton Medical manufactured the stent and signed a strategic distribution agreement with Cardinal Health’s interventional vascular business, Cordis.
“We are actively preparing to commercially launch this product with Tryton to ensure physicians will soon have a new treatment option in their cath labs to help deliver the best patient care available,” David Wilson, president of Cordis, said in March.
During the procedure, the cobalt chromium stent is deployed in the side branch artery using a standard, single wire, balloon-expandable stent delivery system. A standard drug-eluting stent is then placed in the main vessel.
In an investigational clinical trial, the Tryton Side Branch Stent was shown to reduce the need for additional bailout stenting and led to statistically significant lower side branch percent diameter stenosis after a nine-month follow up, when compared with provisional stenting.
To date, the stent has been used to treat over 12,000 patients globally. In addition to the U.S., it’s also commercially available in multiple countries within Europe, the Middle East and Africa.
CAD patients often experience bifurcation, which is a buildup of plaque at the site where one artery branches from another. The standard treatment involves placing a stent in the main branch, but the side branch is usually not stented, leaving it susceptible to complications like occlusion.
"Bifurcation blockages are somewhat more challenging for cardiac interventionalists to treat than blockages that do not involve side-branch vessels, because current stents do not come in a "Y" configuration,” Dr. Antonious Attallah, cardiologist at SJH&MC who performed the procedure, said in a statement.
The Tryton Side Branch Stent, which received FDA approval in February, was designed to provide complete lesion coverage. Tryton Medical manufactured the stent and signed a strategic distribution agreement with Cardinal Health’s interventional vascular business, Cordis.
“We are actively preparing to commercially launch this product with Tryton to ensure physicians will soon have a new treatment option in their cath labs to help deliver the best patient care available,” David Wilson, president of Cordis, said in March.
During the procedure, the cobalt chromium stent is deployed in the side branch artery using a standard, single wire, balloon-expandable stent delivery system. A standard drug-eluting stent is then placed in the main vessel.
In an investigational clinical trial, the Tryton Side Branch Stent was shown to reduce the need for additional bailout stenting and led to statistically significant lower side branch percent diameter stenosis after a nine-month follow up, when compared with provisional stenting.
To date, the stent has been used to treat over 12,000 patients globally. In addition to the U.S., it’s also commercially available in multiple countries within Europe, the Middle East and Africa.