by
Diana Bradley, Staff Writer | November 23, 2011
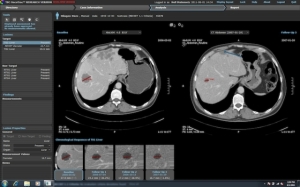
Translational Sciences Corporation's
OncoTrac
On Monday, Translational Sciences Corporation announced FDA 510(k) clearance for commercialization of its medical imaging software OncoTrac, designed to efficiently assess treatment response to breast, lung, colorectal, prostate, and lymphoma metastatic tumors.
"OncoTrac is a fantastic product," said Howard Pinsky, CEO of Cambridge, Mass.-based TSC. "It provides radiologists and oncologists with the opportunity to introduce definitive tumor assessments in patients going through chemotherapy treatment."
Featuring advanced report generation capabilities -- which can be exported and stored in both Picture and Archive Communications Systems and electronic medical records -- the software provides additional value through improved visualization of quantitative response to therapy.
OncoTrac's software utilizes tumor response assessments via criteria including RECIST 1.0, RECIST 1.1, WHO and Choi. Commonly required by clinical trial protocols, this criterion is often submitted as primary and secondary end-point data in new anti-cancer FDA drug approval applications.
Originally developed at the German Cancer Research Center by Mint Medical GmbH, OncoTrac's vendor-neutral platform is currently used under the brand Mint Lesion at major European cancer centers.
Next week's Annual Meeting of the Radiological Society of North America in Chicago will include an OncoTrac demonstration.
